electronic configuration of copper|electron configuration chart : iloilo Since 1s can only hold two electrons the next 2 electrons for magnesium go in . Quickly convert Central European Time (CET) to time in Taipei, Taiwan with this easy-to-use, modern time zone converter.
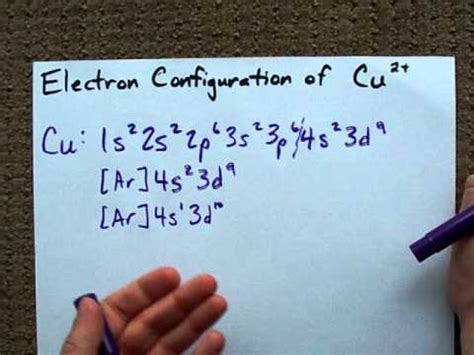
electronic configuration of copper,The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write .In order to write the Calcium electron configuration we first need to know the .Since 1s can only hold two electrons the next 2 electrons for magnesium go in .Copper (Cu, Cu +, Cu 2+) Iron (Fe, Fe 2+, Fe 3+) Read my article in Science .The nex six electrons will go in the 2p orbital. The p orbital can hold up to six .Chlorine (Cl) - Electron Configuration for Copper (Cu, Cu+, Cu2+) - UMDLithium is the third element with a total of 3 electrons. In writing the electron . Learn how to write the electron configuration for copper and its ions using the periodic table and the orbital filling rules. Watch a video explanation with examples and diagrams.electron configuration chart Writing an electron configuration for a transition metal ion starts with the same steps as writing the configuration of an s- or p-block cation: Write the electron .electronic configuration of copper electron configuration chartLearn how copper atoms arrange their electrons across different shells and subshells, and how this affects its valency and properties. Find out the applications and importance of . Learn how to write the electron configuration and orbital notation for copper (Cu) with Mr. Causey's video tutorial. See examples, tips and tricks for transition metals and orbital overlapping. Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for .
What is The Electron Configuration of Copper. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of Cu. If the general pattern of filling electron orbitals is followed, then copper’s electron .
Copper, a transition metal with the symbol Cu, and atomic number 29, is a d-block element in the periodic table. Let us discuss the electronic configuration of Cu. The .Understanding the Electron Configuration of Cu+. Periodic Table. Have you ever wondered how electrons are arranged in copper ions? The electron configuration of Cu+ . Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for copper, 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^9 or in noble gas configuration [Ar] 4s^2 3d^9. However, because the 3d orbital is so much larger then the 4s orbital and the 3d orbital . Writing Electron configuration of Copper. Mr. Causey shows you step by step how to write the electron configuration and orbital notation for copper (Cu). You.Similarly, the observed electron configuration of copper is [Ar] 4s 1 3d 10 instead of [Ar] s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels . In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is . Copper is a chemical element with atomic number 29 which means there are 29 protons and 29 electrons in the atomic structure.The chemical symbol for Copper is Cu. Electron Configuration and Oxidation States of Copper. Electron configuration of Copper is [Ar] 3d10 4s1. Possible oxidation states are +1,2. Electron ConfigurationElectronic configuration: Electronic configuration is the distribution of the electrons in orbitals of atoms using some basic principles like the Pauli exclusion principle and the Aufbau principle. Electronic configuration of Copper: The atomic number of Copper(Cu) = 29; Therefore, the expected electronic configuration is Ar 3 d 9 4 s 2.

That wouldn't be true if the outer electron in potassium was 3d 1. s- and p-block elements. The elements in Group 1 of the Periodic Table all have an outer electronic structure of ns 1 (where n is a number between 2 and 7). All Group 2 elements have an outer electronic structure of ns 2. Elements in Groups 1 and 2 are described as s-block elements. Let us use this smart electron configuration calculator to determine the electron configuration of copper: Choose copper (Cu) from the drop-down list of elements. Check the atomic number (29) and atomic mass (63.546) of copper. Read here how to calculate atomic mass. Read the full electron configuration:
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Copper (Cu) [Ar] 3d 10 4s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1: 2, 8, 18, 1: 30: Electron configuration of Zinc (Zn) [Ar] 3d 10 4s 2: 1s 2 2s 2 2p 6 .Answer 2: Gallium has 31 electrons so the full electronic configuration is: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 1. The shorthand electronic configuration is: [Ar] 3d 10 4s 2 4p 1. Even though the 4s is filled first, the full electron configuration is .In Chromium, its electron configuration is [Ar] 3d^5 4s^1, meaning that one electron from the 4s orbital moves to the 3d orbital. This half-filled 3d orbital provides stability to the atom, leading to increased reactivity and a higher tendency to form compounds. Copper, on the other hand, has an electron configuration of [Ar] 3d^10 4s^1.Electron configuration of copper. The electron configuration of copper is 1s2 2s2 2p6 3s2 3p6 4s2 3d9. Copper is a transition metal that is part of the copper family along with gold and silver. It has a coppery color and a metallic sheen that makes it stand out perfectly. In the table, it corresponds to the symbol Cu, which has atomic number 29.
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and . This chemistry video tutorial covers exceptions in electron configuration using the examples of Chromium and Copper.Electron Configuration - Introduction: .

Similarly, the observed electron configuration of copper is [Ar]4s 1 3d 10 instead of [Ar]s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell.The Electron: Crash Course Chemistry #5. Video 2.6.2 2.6. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on).
electronic configuration of copperThis page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.The electron configuration of Cu+ is 1s22s22p63s23p63d10, where one electron is removed from the 4s orbital. Copper’s electron configuration is an exception to the Aufbau principle, as one of the 4s electrons moves to the 3d subshell. Copper’s d subshell contributes to its distinct properties, such as its reddish-brown color and high .
electronic configuration of copper|electron configuration chart
PH0 · write the electron configuration for lithium
PH1 · noble gas configuration for fe
PH2 · how many 3d electrons are in cu
PH3 · full electron configuration of copper
PH4 · full electron configuration cu
PH5 · electron configuration of all elements
PH6 · electron configuration chart
PH7 · complete electron configuration for gold
PH8 · Iba pa